Abstract
Introduction
Integrase strand transfer inhibitor (InSTI)-based antiretroviral regimens have become the recommended antiretroviral therapy for people living with HIV (PLWH) who are antiretroviral-naïve or stably antiretroviral-treated. This meta-analysis aimed to systematically review the efficacy and safety of coformulated bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) among PLWH.
Methods
Randomized controlled trials (RCTs) were included to compare the efficacy and safety between BIC/FTC/TAF and other antiretroviral regimens containing a non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or integrase strand transfer inhibitor plus two nucleos(t)ide reverse transcriptase inhibitors. A Mantel–Haenszel model was used to investigate the combination or interaction of a group of independent studies. I2 was used to determine whether a fixed-effect model or random-effect model was to be used.
Results
A total of seven published randomized clinical trials including 3547 participants were analyzed; three studies were conducted in antiretroviral-naïve PLWH and four in stably antiretroviral-treated PLWH. At week 48, the efficacy with BIC/FTC/TAF was not statistically significantly different from that with control regimens [odds ratio (OR) 1.01 (95% CI 0.79, 1.30)]. BIC/FTC/TAF had comparable safety profiles to control regimens: OR for all adverse effects (AEs) was 0.92 (95% CI 0.78, 1.09); OR for any grade 3 or grade 4 AEs was 0.96 (95% CI 0.66, 1.39); and OR for treatment-related AEs was 1.31 (95% CI 0.68, 2.53).
Conclusions
This meta-analysis of published randomized clinical trials of antiretroviral-naïve and stably antiretroviral-treated PLWH suggests that BIC/FTC/TAF is as safe and efficacious as are its comparators at week 48. The interstudy differences in selected populations and control regimens may lead to the high heterogeneity of the meta-analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
To analyze the efficacy and safety of the coformulated fixed-dose combination bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) tablet compared to other approved antiretroviral regimens among people living with HIV by systematic meta-analysis of published clinical trial results. |
What was learned from the study? |
At week 48, the overall efficacy with BIC/FTC/TAF was not statistically significantly different from that with control regimens [odds ratio (OR) 1.01 (95% CI 0.79, 1.30)]. |
BIC/FTC/TAF had comparable safety profiles to control regimens: OR for all adverse effects (AEs) was 0.92 (95% CI 0.78, 1.09); OR for any grade 3 or grade 4 AEs was 0.96 (95% CI 0.66, 1.39); and OR for treatment-related AEs was 1.31 (95% CI 0.68, 2.53). |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14437394.
Introduction
Since 1996, highly active antiretroviral therapy (HAART) consisting of two nucleos(t)ide reverse transcriptase inhibitors (NRTIs) plus either a protease inhibitor (PI) boosted with ritonavir or cobicistat, a non-nucleoside reverse transcriptase inhibitor (nNRTI), or an integrase strand transfer inhibitor (InSTI), has become a new treatment paradigm for people living with HIV (PLWH). However, the earlier antiretroviral regimens required taking several different pills at multiple times per day, particularly those regimens for antiretroviral-naïve individuals with pre-treatment or transmitted antiretroviral resistance of HIV-1 as well as antiretroviral-experienced individuals with virologic failure, which might lead to increases in pill burden and decreases in adherence and effectiveness [1]. The International Conference on Antiretroviral Drug Optimization (CADO) suggests that the characteristics of target product profiles of an ideal antiretroviral regimen should comprise safety, efficacy, tolerability, durability, stability, and convenience, and also be suitable for special populations and at lower costs for treatment [2].
The evolution of HIV therapeutics has been marked by a shift from multiple-tablet regimens that need to be taken several times per day to once-daily, single-tablet regimens with fixed-dose combination (FDC). Several FDC regimens have become available for the treatment of HIV-1 infection, which include coformulated efavirenz, emtricitabine, and tenofovir disoproxil fumarate (EFV/FTC/TDF); dolutegravir, lamivudine, and abacavir (DTG/3TC/ABC); rilpivirine, FTC, TDF (RPV/FTC/TDF); elvitegravir, cobicistat, FTC, TDF (EVG/c/FTC/TDF); EVG, cobicistat, FTC, and tenofovir alafenamide (EVG/c/FTC/TAF); RPV/FTC/TAF; DTG/RPV; bictegravir, FTC, and TAF (BIC/FTC/TAF); and DTG/3TC [1]. Most of the international guidelines on antiretroviral treatment for adults and adolescents living with HIV recommend FDC regimens as the first-line regimens for antiretroviral-naïve patients and as stable switch regimens for suitable patients who have achieved viral suppression. In both updated Department of Health and Human Service (DHHS) guidelines in February 2021 and European AIDS Clinical Society (EACS) guidelines in October 2020 [3, 4], two single-tablet three-drug regimens (BIC/FTC/TAF and DTG/3TC/ABC) and one two-drug regimens (DTG/3TC) containing InSTI are the approved combinations for initial treatment of PLWH.
The aim of this systematic review was to analyze the efficacy and safety of the coformulated FDC BIC/FTC/TAF tablet compared to other approved antiretroviral regimens among PLWH.
Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for the reporting of systematic reviews and meta-analysis.
Search Strategy
PubMed, Embase, and Medline Library databases were searched up to December 2020 to identify randomized controlled trials (RCTs) that were undertaken for any purpose (equivalence, superiority, or noninferiority), at a single or multiple centers, in any country, and with any follow-up duration. PubMed, Embase, and Medline were searched with the use of the keywords “Biktarvy®”, or “BIC/FTC/TAF”, or “B/F/TAF”, or “bictegravir/emtricitabine/tenofovir alafenamide”, and “randomized controlled trial”, and “HIV-1”. Reference lists of identified studies and major reviews, abstracts of conference proceedings, scientific meetings, and clinical trials registries (www.clinicaltrials.gov) were searched.
No limitation was used during the literature search. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Research ethics committee approval was waived because this study did not involve any human participants or animals.
Eligibility Criteria
Eligible study for this meta-analysis met the following criteria:
-
The study was designed as an RCT.
-
Outcomes of interest were efficacy and safety assessment among PLWH participating in the trials.
-
Comparisons were conducted between BIC/FTC/TAF and antiretroviral regimens containing nNRTI, PI, or InSTI plus two NRTIs.
Outcomes
Primary outcome:
-
The proportion of subjects with plasma HIV-1 RNA < 50 copies/mL at week 48 according to the US Food and Drug Administration (FDA) snapshot algorithm [5]
Secondary outcomes:
-
The change from baseline in CD4 cell count and CD4 percentage at week 48
-
Safety profile at week 48
Data Extraction
For each eligible study, the main categories were based on the following: setting, details of participants (number and baseline characteristic by group), details of the study (study design, type, and duration of follow-up), details of the control group included, primary and secondary outcome description, and outcome measures, safety and tolerability, number of withdrawals or discontinuations in each group with reasons. Data at weeks 48 and 96 were considered.
Statistical Analysis
The information on BIC/FTC/TAF efficacy from the RCTs was analyzed using a Mantel–Haenszel model to investigate the combination or interaction of a group of independent studies. Clinical heterogeneity was explored, and statistical heterogeneity was assessed using Cochrane’s Q test to calculate the weighted sum of squared differences between individual study effect and pooled effect across studies. The heterogeneity was examined using the I2 statistic for the percentage of variation across the studies that was due to heterogeneity rather than chance, with I2 of 0% as no heterogeneity, 25% as low heterogeneity, 50% as moderate heterogeneity, and 75% as high heterogeneity [6]. When I2 was less than 50%, studies were pooled using a fixed-effect model, whereas, when I2 was greater than 50%, random-effect model was used. The p value of BIC/FTC/TAF versus other control treatment groups and the 95% confidence interval (CI) were analyzed using data extracted from controlled studies. The bias risk tool was provided by the Cochrane Collaboration to evaluate the quality of the literature using RevMan software 5.3 [7].
For the continuous outcome when the authors only provided the median, first and third quartiles of data, but not mean and standard deviation from the reported summary data, the quantile estimation (QE) method originally introduced by McGrath et al. was applied to estimate the sample mean and standard deviation [8].
Results
Overview of Literature Search
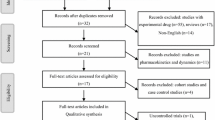
The search of the three databases identified 115 titles from the initial evaluation, of which 12 were duplicated and discarded, and 59 were not relevant to review question and discarded, resulting in 44 unique citations. The full text was obtained for seven published studies that were examined in detail, with 37 articles excluded due to either being not RCTs, duplicated or overlapped data in multiple records, not investigating main outcomes of interest, or no available results published because the trials were not yet completed. The remaining seven studies were included in this systematic review and meta-analysis and the PRISMA flow chart of the searching process is described in Fig. 1. All included studies were based on moderate- to high-quality evidence. Table 1 provides a brief description of the details of the seven included studies. The RCTs listed in Table 1 were non-inferiority studies where the non-inferiority margin was 0.12 for GS-US-141-1475, GS-US-380-1489, and GS-US-380-1490 and 0.04 for GS-US-380-1844, GS-US-380-1878, GS-US-380-1961, and GS-US-380-4030 according to US Food and Drug Administration (FDA) guidance for antiretroviral-naïve populations and virologically suppressed populations, respectively [5].
Study Characteristics
A total of seven studies that included 3547 participants were analyzed in this review. Further description by main eligibility criteria is summarized in Table 2. Three double-blinded studies enrolled 1372 antiretroviral-naïve HIV-1-infected participants who were randomized to either BIC/FTC/TAF or control groups (DTG + FTC/TAF or DTG/3TC/ABC). Four studies enrolled 2175 antiretroviral-treated participants who had achieved viral suppression and were randomized to either BIC/FTC/TAF or control groups (DTG/3TC/ABC or remaining on current treatment). All studies assessed virologic outcomes at week 48. The median age ranged from 31.5 to 50.5 years and approximately 24.3% of the participants were female. In GS-US-380-1961, only female subjects were recruited.
Efficacy Profile of BIC/FTC/TAF Versus Other Control Groups at Week 48
Efficacy data for BIC/FTC/TAF and control groups were extracted from the seven selected RCTs and the primary endpoint at week 48. Figure 2 shows a forest plot of the results of the meta-analysis. In part 1.1.1 of Fig. 2, the I2 of the meta-analysis was 18% for the seven selected studies, indicating that the studies were considered of very low heterogeneity to each other and, therefore, a fixed model was used for the meta-analysis. Mantel–Haenszel fixed effect of odds ratio (OR) was 1.01 (95% CI 0.79, 1.30; p = 0.92), which indicates that the differences in terms of overall efficacy between BIC/FTC/TAF and control groups were not statistically significant.
A funnel plot of the selected studies according to a log (OR) distribution for the efficacy data was symmetrical, which demonstrated a lack of publication bias in this meta-analysis, as shown in Supplementary Fig. 1 (Fig. S1). GS-US-141-1475 [9] is located at the very right side of the funnel plot because of the small sample size of the study, which was more heterogenous compared to other selected studies. As a result of the presence of heterogeneity, GS-US-141-1475 [9] was excluded from further subgroup analysis described in part 1.1.2 of Fig. 2 for antiretroviral-naïve participants. In part 1.1.2, studies that recruited antiretroviral-naïve participants demonstrated non-inferiority in the efficacy of BIC/FTC/TAF compared to the active controlled group, for which the OR was 0.75 (95% CI 0.50, 1.13; p = 0.17). Similarly, the subgroup analysis for studies enrolling virologically suppressed participants indicates non-inferiority in efficacy of BIC/FTC/TAF compared to that of the active controlled group, with an OR of 1.19 (95% CI 0.86, 1.66; p = 0.29) as shown in part 1.1.3 of Fig. 2.
The overall results of the efficacy assessment between BIC/FTC/TAF and active controls in this meta-analysis suggests that the FDC of BIC/FTC/TAF has high antiretroviral efficacy in both antiretroviral-naïve and virologically suppressed populations. These meta-analysis results were consistent with the results of individual RCTs, which have demonstrated that BIC/FTC/TAF was non-inferior to active controls. Figure 3 provides the forest plot of the treatment difference for all included studies, except GS-US-141-1475 [9], owing to the lack of available data, which indicated that all of the selected studies had its lower limit bound stay within the non-inferiority margin as defined by the US FDA snapshot algorithm.
Change of CD4 Cell Count at Week 48
The changes of CD4 cell count from baseline to week 48 are also visualized by forest plot as shown in Fig. S2. The random effect model was used because the I2 was greater than 50%. The results from seven selected studies showed no significantly different changes of CD4 count at week 48, with a mean difference of − 2.86 cells/mm3 (95% CI − 20.27, 14.55; p = 0.75). However, greater changes of CD4 count were observed in GS-US-141-1475 [9], GS-US-380-1489 [10], and GS-US-380-1490 [11], which included antiretroviral-naïve participants. In GS-US-380-1844 [12], GS-US-380-1878 [13], GS-US-380-1961 [14], and GS-US-380-4030 [15] that recruited virologically suppressed participants, the changes of CD4 count were lower than those in antiretroviral-naïve participants.
Safety Profile of BIC/FTC/TAF Versus Other Control Groups
The comparisons of safety profile between BIC/FTC/TAF and other control groups were analyzed including all adverse events (AEs), any grade 3 or grade 4 AE occurring during the treatment period, and treatment-related AEs shown in Fig. S3a, b, and c, respectively. Fixed-effect model was used for meta-analysis in all AE analysis because of low heterogeneity being noted (I2 = 25%). Any grade 3 or grade 4 AE and treatment-related AEs were analyzed by random-effect model due to the fact that the I2 was greater than 50% (72% and 90%, respectively), indicating the heterogeneity between the selected studies.
The comparisons of safety profile between BIC/FTC/TAF and selected control groups shown in Figs. S3a, b, c indicated the safety concerns neither favored BIC/FTC/TAF group or selected control groups as the OR was 0.92 (95% CI 0.78, 1.09; p = 0.34) for all AE analysis, 0.96 (95% CI 0.66, 1.39; p = 0.83) for any grade 3 or grade 4 AE analysis, and 1.31 (95% CI 0.68, 2.53; p = 0.42) for treatment-related AE analysis. Although the data did not demonstrate statistically significant differences in subgroup analysis in terms of safety assessment, the analyses of overall AE and any grade 3/4 AE (Fig. S3a and b, respectively) showed a trend of lower safety concerns with BIC/FTC/TAF compared with active control group, whereas the analysis of treatment-related AE (Fig. S3c) showed a trend of higher safety concerns with BIC/FTC/TAF, which may be due to the observation bias related to the open-label study design.
Weight Changes of BIC/FTC/TAF Versus Control Group from Baseline
As limited available data for weight changes from baseline were published, only week 144 data for GS-US-380-1489 [10] and GS-US-380-1490 [11] and week 48 data for GS-US-380-4030 [15] were included in this meta-analysis. The QE method was used to estimate the mean and standard deviation from the given median and IQR found in the published studies [15, 16]. From the given estimated mean of each group as presented in Fig. S4, the weight increase was more evident in antiretroviral-naïve participants compared to virologically suppressed participants. The overall weight gain noted for both BIC/TFC/TAF and control group was not statistically significant with a mean difference of 0.17 kg (95% CI − 0.30, 0.64; p = 0.48).
Discussion
BIC/FTC/TAF (Biktarvy®, GS-9883) is an oral single-table regimen approved for once-daily treatment of HIV-1 infection in adults with no known substitution associated with resistance to the individual component. We performed a systematic review on all published clinical trial data of BIC/FTC/TAF and carried out a meta-analysis on the virologic outcome of those studies. The endpoint of the trials was the proportion of participants in each treatment group that achieved plasma HIV RNA < 50 copies/mL at week 48. On the basis of the meta-analysis, treatment with BIC/FTC/TAF and with active control group led to similarly high levels of viral suppression at week 48 (OR 1.01; 95% CI 0.79, 1.30) and there were no significant differences in terms of changes of CD4 cell count given the mean difference of 2.86 cells/mm3 (95% CI − 20.27, 14.55). Safety assessment indicated no safety benefit favoring either BIC/FTC/TAF or active control group, given the OR of 0.92 (95% CI 0.78, 1.09) for all AE analysis, 0.96 (95% CI 0.66, 1.39) for any grade 3 or grade 4 AE analysis, and 1.31 (95% CI 0.68, 2.53) for treatment-related AE analysis.
BIC/FTC/TAF is a complete regimen for treatment of HIV-1 infection in adults who are antiretroviral-naïve or to replace the current antiretroviral regimen in those who are virologically suppressed. The efficacy results in subgroup analysis are consistent with the non-inferiority to DTG-based therapy (DTG/3TC/ABC or DTG + FTC/TAF) in achieving virologic suppression in antiretroviral-naïve adults at 48 weeks (OR 0.75; 95% CI 0.50, 1.13). Similarly, BIC/FTC/TAF was non-inferior to ongoing DTG/3TC/ABC or boosted EVG- or PI-based therapy in preventing virologic rebound over 48 weeks in antiretroviral-treated participants (OR 1.19; 95% CI 0.86, 1.66).
Despite the similar safety profile between BIC/FTC/TAF and active control groups, the bothersome symptoms of the participants assessed using the HIV-Symptom Index (HIV-SI) in GS-US-380-1489 and GS-US-380-1844 revealed that some symptoms were less frequent with BIC/FTC/TAF than with DTG/3TC/ABC over 48 weeks’ treatment, with the most notable differences being in reports of fatigue/loss of energy, nausea/vomiting, dizziness/light-headedness, and difficulty sleeping in adjusted logistic regression analyses [17]. The most common treatment-related AEs with BIC/FTC/TAF included headache, diarrhea, and nausea in the included studies. Furthermore, no participants receiving BIC/FTC/TAF who had viral rebound developed treatment-emergent resistance-associated mutations (RAMs) in all included studies, indicating that BIC/FTC/TAF demonstrated a high resistance barrier. In GS-US-141-1475 [9], one participant with emergence of T97A InSTI mutation was seen in the DTG group, while one participant developed M184M/I/V resistance while on EVG/c/FTC/TAF regimen in GS-US-380-1961 [14]. BIC also displayed in vitro activity against HIV-1 strains with mutations conferring resistance to EVG and raltegravir (RAL), with 50% maximal effective concentration (EC50) values of < 5 nmol/L for all viruses with single InSTI RAMs (e.g., Y143R, Q148R) and < 5 or < 10 nmol/L for the majority of those with double or triple InSTI RAMs (e.g., E92Q/N155H ± G163R) [18].
Weight gain was more evident during the first 2 years of the initiation of antiretroviral therapy for antiretroviral-naïve PLWH in this meta-analysis, which was consistent with the recent pooled analysis of eight RCTs [19]. Black race and female sex have been noted to be associated with greater weight gain. The association between weight gain and low CD4 count and high plasma HIV RNA at baseline in the pooled analysis suggests the return-to-health phenomenon. Weight gain with InSTI-, DTG-, and BIC-based regimens was greater than that with NNRTI- or PI-based regimens; the causes for the differences observed remain unclear. The well-tolerated and easier-to-take characteristics of InSTI-based regimens might be contributory.
The heterogeneity noted in AE and CD4 assessments between studies may be due to the following factors. First, different regimens were selected for active control arm (Table 1). Second, different populations were targeted, antiretroviral-naïve vs stably antiretroviral-treated population. Third, there were two different study designs, double-blinded vs open-label. The more frequently reported AE in the switch arm (BIC/FTC/TAF) may have been influenced by the open-label study design as participants who were stable and tolerating their baseline regimens were less likely to report symptoms or to attribute these symptoms to study drugs than those who switched to a new regimen. Fourth, GS-US-380-1961 [14] only recruited HIV-positive women whereas other studies recruited both adult male and female subjects. Moreover, the different proportions of female subjects ranged from 5.5% to 17%, which may also lead to some degree of heterogeneity. Last, the baseline conditions between the selected studies varies. For instance, some studies allowed recruitment of subjects with both chronic infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) or advanced HIV infection, but others only recruited relatively healthy populations with good renal function.
Current HIV treatment guidelines recommend regimens containing an InSTI plus two NRTIs as the first-line treatment. However, individual InSTIs have different characteristics that may influence the treatment choice, and not all available InSTIs are appropriate for some patients, compared with other antiretrovirals from other antiretroviral classes. For example, RAL must be dosed 400 mg twice daily and is not coformulated with other antiretrovirals as a complete regimen. EVG is dosed once daily when given with a pharmacokinetic booster and is available as a complete regimen when coformulated with cobicistat, FTC, and either TDF or TAF. DTG is coformulated with ABC and 3TC as a single tablet, but ABC is not appropriate in individuals positive for HLA-B*5701 and has been linked to increased risk of cardiovascular events in several epidemiological studies [20, 21]. Moreover, exposure to DTG appears to confer a higher risk of neural tube defect in newborns of HIV-positive mothers than other regimens or HIV-negative mothers, though there are insufficient data for pregnant women with exposure to BIC/FTC/TAF [22]. Overall, the FDC of BIC/FTC/TAF, containing an unboosted, once-daily InSTI, has encompassed the ideal characteristics of the target drug profile of safety, efficacy, tolerability, durability, stability, and convenience, and is suitable for most HIV-infected populations.
Currently, several RCTs with BIC/FTC/TAF targeting special populations are registered with ClinicalTrials.gov for further investigations on the safety, tolerability, and efficacy. GS-US-380-4458 (NCT03547908) is evaluating the safety and tolerability of HIV/HBV-coinfected patients. Furthermore, the median age of the study populations included in this meta-analysis is 40.2 years, and sparse data are available on the efficacy and safety in PLWH who are aged 65 years and older or those who are aged 18 or younger. In GS-US-380-4449 that recruited 86 virologically suppressed elderly patients (median age 69 years), the virological suppression rate was 90.7% [23]. The phase 1b study of GS-US-380-5310 (NCT03960645) is registered to target virologically suppressed, pregnant women in their second and third trimesters. Since the latter study is still in the recruitment period, it was not included in this meta-analysis. In VIKING-3, a single-arm, open-label phase III study, DTG had demonstrated short- and long-term antiviral activity in subjects with integrase resistance [24]. Future research of BIC/FTC/TAF in PLWH with HIV-1 harboring integrase resistance or in antiretroviral-experienced PLWH who are viremic is needed.
There are several weaknesses of BIC/FTC/TAF. First, BIC is a substrate of CYP3A and UGTA1A1 and, coadministration with strong inducers of CYP3A and UGT1A1, such as St. John’s wort, rifampin, and rifapentine, can lead to significant decreases of plasma concentration of BIC [25]. Second, BIC inhibits organic cation transporter 2 (OCT2) and multidrug and toxin extrusion transporter 1 (MATE1); therefore, the plasma concentrations of coadministered drugs, such as dofetilide, that are substrates of OCT2 and MATE1 may increase. Third, the safety data on the birth outcome of pregnant women in the first trimester remains sparse. World Health Organization (WHO) guidelines published in July 2019 stated that, because of the declining incidence of neural tube defect with the increasing number of included pregnant women and the benefit outweighs the risk, DTG remains the preferred antiretroviral drug for the first- and second-line regimens for pregnant women [26].
Although this meta-analysis demonstrates the similar efficacy, safety, and tolerance profile of BIC/FTC/TAF compared to the selected control treatments, there are some limitations in common from the selected RCTs. All the trials were powered for the primary efficacy endpoint at 48 weeks of treatment, but not powered for secondary outcomes. Therefore, the treatment-related AEs of BIC/FTC/TAF, such as central nervous system events seen in participants treated with DTG, might only become apparent after longer durations of clinical use or in broader patient populations. The eligible participants were relatively healthy and only a small proportion of study participants had advanced HIV disease. The female population remained under-represented in all included studies except for GS-US-380-1961. The proportions of participants with chronic HCV or HBV coinfection were small. All the studies included in this meta-analysis are sponsored by the respective drug manufacturer. Also, five of the included studies are double-blinded design, in which the pill burden for the participants in those studies was greater than one pill because of the placebo drug, which does not reflect the real-world experience with single-tablet FDC BIC/FTC/TAF. On the basis of the aforementioned limitations, phase IV post-marketing surveillance studies are warranted to further monitor boarder patient populations with a longer safety follow-up duration, and also to evaluate the real-world applicability of evidence derived from RCTs.
Conclusions
This meta-analysis of seven published clinical trials suggests that, at week 48, BIC/FTC/TAF is as safe and efficacious as are its comparators among antiretroviral-naïve and stably antiretroviral-treated PLWH.
References
Vitoria M, Rangaraj A, Ford N, Doherty M. Current and future priorities for the development of optimal HIV drugs. Curr Opin HIV AIDS. 2019;14(2):143–9.
Crawford KW, Ripin DHB, Levin AD, Campbell JR, Flexner C. Optimising the manufacture, formulation, and dose of antiretroviral drugs for more cost-efficient delivery in resource-limited settings: a consensus statement. Lancet Infect Dis. 2012;12:550–60.
Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV, 2021. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Accessed 02 April 2021.
European AIDS Clinical Society. EACS Guidelines 10.1, 2020. https://www.eacsociety.org/files/guidelines-10.1_5.pdf. Accessed 02 April 2021.
US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Human immunodeficiency virus-1 Infection: developing antiretroviral drugs for treatment-guidance for industry, 2015. https://www.fda.gov/media/86284/download. Accessed 02 April 2021.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327(7414):557–60.
Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for accessing risk of bias in randomised trial. BMJ. 2011;343:d5928.
McGrath S, Zhao XF, Steele R, Thombs BD, Benedetti A, Depression Screening Data (DEPRESSD) Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29(9):2520–37.
Sax PE, Dejesus E, Crofoot G, et al. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. Lancet HIV. 2017;4(4):e154–60.
Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390(10107):2063–72.
Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390(10107):2073–82.
Molina JM, Ward D, Brar I, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adult with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV. 2018;5(7):e357-365.
Daar ES, Dejesus E, Ruane P, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3 non-inferiority trial. Lancet HIV. 2018;5(7):e347-356.
Kityo C, Hagins D, Koenig E, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) in virologically suppressed HIV-1 infected women: a randomized, open-label, multicenter, active-controlled, phase 3, noninferiority trial. J Acquir Immune Defic Syndr. 2019;82(3):321–8.
Sax PE, Rockstroh JK, Luetkemeyer AF, et al. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa988.
Orkin C, Sax PE, Arribas JR, et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: week 144 results from two randomized, double-blind, multicentre, phase 3, non-inferiority trials. Lancet HIV. 2020;7:e389-400.
Wohl D, Clarke A, Maggiolo F, et al. Patient-reported symptoms over 48 weeks among participants in randomized, double-blind, phase III non-inferiority trial of adults with HIV on co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus co-formulated abacavir, dolutegravir, and lamivudine. Patient. 2018;11(5):561–73.
Tsiang M, Jones GS, Goldsmith J, et al. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother. 2016;60(12):7086–97.
Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71(6):1380–9.
Young J, Xiao Y, Moodie EEM, et al. Effect of cumulating exposure to abacavir on the risk of cardiovascular disease events in patients from the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2015;69(4):413–21.
Marcus JL, Neugebauer RS, Leyden WA, et al. Use of abacavir and risk of cardiovascular disease among HIV-infected individuals. J Acquir Immune Defic Syndr. 2016;71(4):413–9.
De Ven NSV, Pozniak AL, Levi JA, et al. Analysis of pharmacovigilance database for dolutegravir safety in pregnancy. Clin Infect Dis. 2020;70(12):2599–606.
Maggiolo F, Rizzardini G, Molina JM, et al. Bictegravir/emtricitabine/tenofovir alafenamide in virologically suppressed people with HIV aged ≥ 65 years: week 48 results of a phase 3b, open-label trial. Infect Dis Ther. 2021. https://doi.org/10.1007/s40121-021-00419-5.
Castagna A, Maggiolo F, Penco G, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir- resistant HIV-1: 24-Week results of the phase III VIKING-3 study. J Infect Dis. 2014;210(3):354–62.
Biktarvy [package insert]. Foster City, CA: Gilead Sciences. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210251s000lbl.pdf. Accessed 02 April 2021.
Policy brief: Update of recommendations on first- and second-line antiretroviral regimen. 2019. https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf. Accessed 02 April 2021.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors' Contributions
I.-W.C. and C.-C.H. conceived and designed the study; I.-W.C. conduct the systematic review of meta-analysis and drafting the article. C.-C.H. and H.-Y.S. revised the manuscript and gave the final approval of the manuscript.
Disclosures
Chien-Ching Hung has received research support from Gilead Sciences, Merck, and ViiV and speaker honoraria from Gilead Sciences and ViiV, and served on advisory boards for Gilead Sciences and ViiV. Hsin-Yun Sun has received research support from Gilead Sciences. I-Wen Chen declared no conflict of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chen, IW., Sun, HY. & Hung, CC. Meta-Analysis of Efficacy and Safety of Coformulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide Among People Living with HIV. Infect Dis Ther 10, 1331–1346 (2021). https://doi.org/10.1007/s40121-021-00449-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00449-z