Abstract
Background
The gut incretin hormones GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic peptide) are secreted by enteroendocrine cells following food intake leading to insulin secretion and glucose lowering. Beyond its metabolic function GIP has been found to exhibit direct cardio- and atheroprotective effects in mice and to be associated with cardiovascular prognosis in patients with myocardial infarction. The aim of this study was to characterize endogenous GIP levels in patients with acute myocardial infarction.
Methods and results
Serum concentrations of GIP were assessed in 731 patients who presented with clinical indication of coronary angiography. Circulating GIP levels were significantly lower in patients with STEMI (ST-elevation myocardial infarction; n=100) compared to clinically stable patients without myocardial infarction (n=631) (216.82 pg/mL [Q1–Q3: 52.37–443.07] vs. 271.54 pg/mL [Q1–Q3: 70.12–542.41], p = 0.0266). To characterize endogenous GIP levels in patients with acute myocardial injury we enrolled 18 patients scheduled for cardiac surgery with cardiopulmonary bypass and requirement of extracorporeal circulation as a reproducible condition of myocardial injury. Blood samples were drawn directly before surgery (baseline), upon arrival at the intensive care unit (ICU), 6 h post arrival to the ICU and at the morning of the first and second postoperative days. Mean circulating GIP concentrations decreased in response to surgery from 45.3 ± 22.6 pg/mL at baseline to a minimum of 31.9 ± 19.8 pg/mL at the first postoperative day (p = 0.0384) and rose again at the second postoperative day (52.1 ± 28.0 pg/mL).
Conclusions
Circulating GIP levels are downregulated in patients with myocardial infarction and following cardiac surgery. These results might suggest nutrition-independent regulation of GIP secretion following myocardial injury in humans.
Similar content being viewed by others
Introduction
The gut incretin hormones GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic peptide) are secreted by enteroendocrine cells following nutrient intake leading to insulin secretion and glucose control [1, 2]. Pharmacological activation of the incretin axis is currently used for the treatment of patients with type 2 diabetes [3]. Beyond their glucoregulatory function GLP-1 receptor agonists exert pleiotropic vascular- and cardioprotective effects in different organ systems [4]. For example, we and others found GLP-1 to reduce and stabilize atherosclerotic lesions in ApoE−/− mice by diminishing vascular inflammation [5, 6]. Importantly, six large clinical trials showed improved cardiovascular outcomes in diabetic patients at high cardiovascular risk after treatment with GLP-1-receptor agonists on top of standard antidiabetic therapy [7,8,9,10,11,12]. In contrast, the role of the other incretin hormone GIP for cardiovascular disease (CVD) remains largely unknown. Endogenous GIP stimulates glucose-dependent insulin secretion more potently than GLP-1 [13, 14]. This effect is however lost in patients with diabetes [15, 16]. Further, GIP suppresses glucagon secretion in states of hyperglycemia while stimulating glucagon release in hypoglycemic situations [17]. Recent efforts have led to the development of combined GLP-1/GIP receptor co-agonists which in clinical trials were importantly found to have more potent blood glucose lowering and body weight reducing effects than sole GLP-1 receptor agonists [18]. Cardiovascular outcome trials (i.e. SURPASS-CVOT; NCT04255433) investigating whether the superiority of Tirzepatide vs. GLP-1 receptor agonists can be translated into improved cardiovascular prognosis are ongoing. Posthoc-Analyses of recent clinical trials yielded encouraging results by showing that the reduction in cardiovascular risk markers as hsCRP, MCP-1 and ICAM-1 was stronger by dual GIP and GLP-1 receptor agonism vs. sole GLP-1 receptor agonism [19]. Understanding the cardiovascular functionality of GIP therefore seems of great interest. First experimental studies suggested GIP to have beneficial cardiovascular effects in rodents [20,21,22,23]. Recently we found circulating GIP levels in patients with acute myocardial infarction to be associated with cardiovascular prognosis. In these patients lower GIP levels independently predicted adverse outcome (cardiovascular death) [24]. These findings might suggest a cardiovascular protective role of the endogenous GIP system. The aim of this study was to characterize endogenous GIP levels in patients with acute myocardial infarction.
Methods
Clinical study I
We analyzed blood samples from 731 patients of our cardiovascular biobank (559 male and 172 female), who underwent coronary angiography at the University Hospital Aachen (Department of Cardiology). The only exclusion criterion was failure to give written informed consent. Patients were divided into two groups—patients with acute myocardial infarction (STEMI = ST-elevation myocardial infarction; n = 100) compared to clinically stable patients without myocardial infarction (n = 631). Of all patients with STEMI (n = 100) two patients died within 2 days after enrollment and were classified as patients with fatal acute myocardial infarction. Blood was collected in a random non-fasting manner. After centrifugation at 2000 g at 4 °C for 20 min, serum aliquots of 1 mL were frozen immediately at −80 °C. Total GIP serum levels were determined by using a commercial ELISA kit (Millipore) according to the manufacturers’ instructions. Study protocols and biosampling were approved by the local ethics committee (RWTH University Hospital Aachen) and conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Clinical study II
We enrolled 18 patients (11 male, 7 female) scheduled for cardiac surgery with cardiopulmonary bypass and requirement of extracorporeal circulation as a reproducible condition of myocardial injury at the University Hospital of Munich (Campus Grosshadern). Patients were excluded from the study if they met the following criteria: failure to give written informed consent, pregnancy, diabetes mellitus, fasting glucose >126 mg/dL, use of antidiabetic medication or glucocorticoids.
Blood samples were drawn directly before surgery (baseline), upon arrival at the intensive care unit (ICU), 6 h post arrival to the ICU and at the morning of the first and second postoperative days.
Patients were fasted since the evening of the preoperative day. Glucose levels were assessed on an hourly basis and insulin-infusion rate consequently adjusted to maintain glucose levels between 80 and 126 mg/dL. No glucose containing solutions were given during the day of the procedure, while all patients received continuous infusion of glucose 10% with a rate of 10 ml/h at the morning of the first postoperative day. No additional parenteral or enteral nutrition was administered during the observation period. After centrifugation at 2000g at 4 °C for 20 min, serum aliquots of 1 mL were frozen immediately at −80 °C. Total GIP serum levels were determined by using a commercial ELISA kit (Millipore) according to the manufacturers’ instructions. Study protocols and biosampling were approved by the local ethics committee (University Hospital of Munich, Ludwig-Maximilians-University) and conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Statistical analysis
Continuous data are presented as mean ± SD or SEM and median (Q1-Q3) in case of heavily skewed data. Categorical outcomes are shown as absolute (No.) and relative frequencies (%). A Wilcoxon rank-sum test was performed to analyze the group GIP levels in Clinical study I. In addition, a linear mixed model was computed to adjust for influences of parameters significantly correlated with GIP. The association between GIP and other characteristics was assessed using the Spearman correlation coefficient ρ. To analyze time effects in Clinical study II, a linear mixed model for logarithmized GIP values with time point as an independent factor, a random patient effect and a compound symmetry covariance structure was computed. P-values for the comparison between time points 2–5 to 1 (baseline) were adjusted using the Dunnett–Hsu adjustment for multiple comparisons. The level of significance was set at 5%. Statistical analyses were performed with SAS software version 9.4 (PROC GLIMMIX, PROC NPAR1WAY, PROC MIXED; SAS Institute, Cary NC, USA).
Results
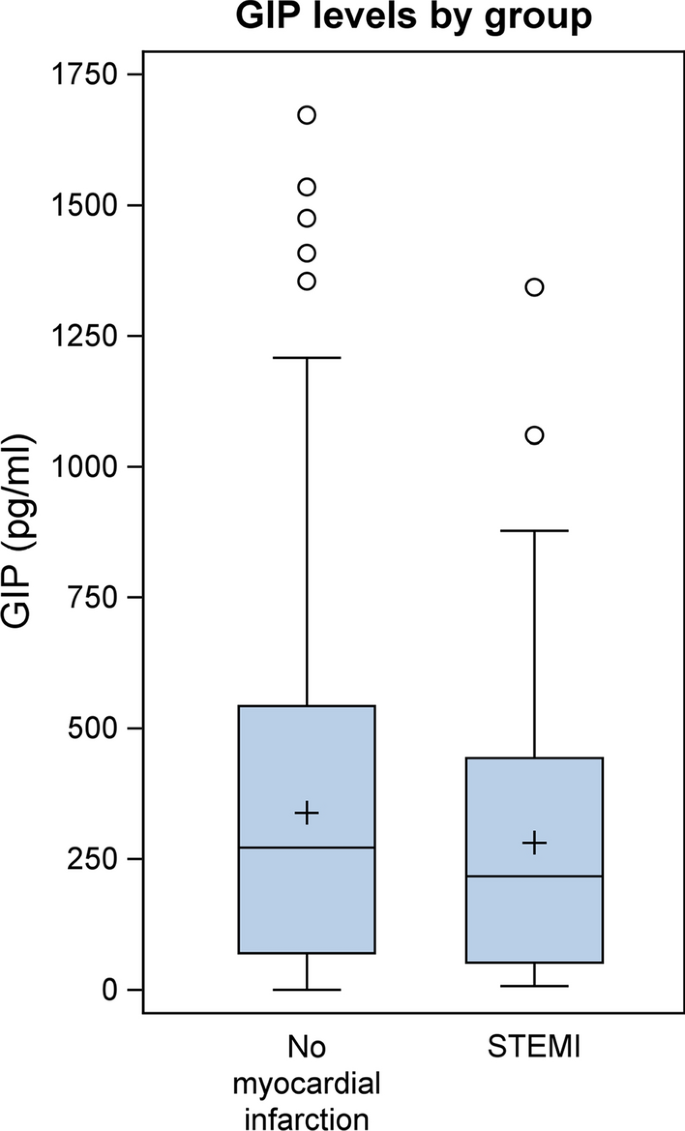
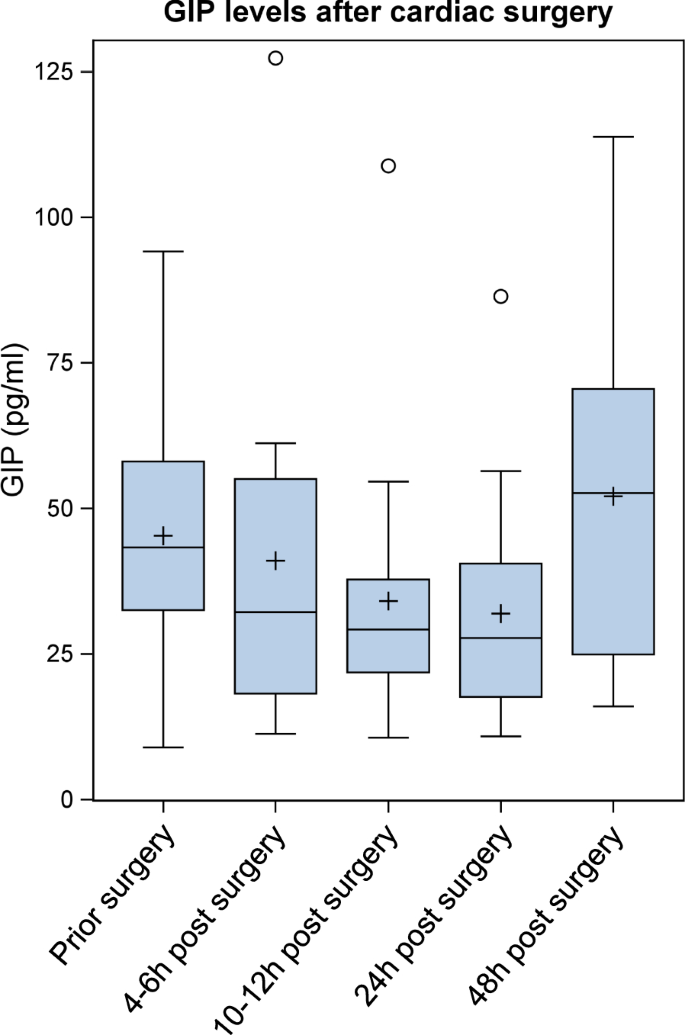
Clinical and laboratory baseline characteristics and biomarker concentrations are shown in Table 1. The study population comprised 731 patients of our cardiovascular biobank (559 male and 172 female), who underwent coronary angiography at the University Hospital Aachen (Department of Cardiology). Patients were divided into two groups—patients with acute myocardial infarction (STEMI = ST-elevation myocardial infarction; n = 100) compared to clinically stable patients without myocardial infarction (n = 631). GIP levels were not associated with age, sex, kidney function, hypertension, creatine kinase, troponin or WBC (white blood cells) while we observed a significant correlation of GIP levels with BMI (body-mass-index), CRP (C-reactive protein) and type 2 diabetes (Table 2). As shown in Fig. 1; Table 1 circulating GIP levels were significantly lower in patients with STEMI compared to clinically stable patients without myocardial infarction (216.82 pg/mL [Q1–Q3: 52.37–443.07] vs. 271.54 pg/mL [Q1–Q3: 70.12–542.41], p = 0.0266). This association remained significant in a multivariable model after adjustment for all parameters which significantly correlated with GIP levels (BMI, CRP and type 2 diabetes) (p = 0.0311). To further elucidate the underlying mechanisms of downregulated GIP levels during acute myocardial infarction we prospectively enrolled 18 non-diabetic patients (11 male, 7 female) scheduled for cardiac surgery with cardiopulmonary bypass and requirement of extracorporeal circulation as a second reproducible condition of myocardial injury. Blood samples were drawn directly before surgery (baseline), upon arrival at the intensive care unit (ICU), 6 h post arrival to the ICU and at the morning of the first and second postoperative days. Mean circulating GIP concentrations decreased in response to surgery from 45.3 ± 22.6 pg/mL at baseline to a minimum of 31.9 ± 19.8 pg/mL at the first postoperative day (p = 0.0384) and rose again at the second postoperative day (52.1 ± 28.0 pg/mL) (Fig. 2).
Serum GIP levels are downregulated in patients with acute myocardial infarction (Clinical study I): Circulating serum GIP levels from patients with acute myocardial infarction (STEMI; n = 100) compared to clinically stable patients without myocardial infarction (n = 631). A Wilcoxon rank-sum test was performed (p = 0.0266)
Serum GIP levels are decreased in response to cardiac surgery (Clinical study II): Kinetics of serum GIP levels over time after cardiac surgery (time point 1: at baseline before cardiac surgery, time point 2: at arrival to the ICU (4–6 h post initiation of surgery), time point 3: 6 h post arrival to the ICU (10–12 h post initiation of surgery), time points 4 and 5: in the morning one and two days after surgery). A linear mixed model for logarithmized GIP values with time point as independent factor, a random patient effect and a compound symmetry covariance structure was computed (time point effect p = 0.0105). P-values for the comparison between time points 2– to 1 (baseline) were adjusted using the Dunnett-Hsu adjustment for multiple comparisons (comparison between time point 4 and baseline p = 0.0384)
Discussion
Under physiological conditions GIP is secreted from enteroendocrine K-cells in the gut following food ingestion [25]. This study demonstrates that circulating GIP levels are reduced in patients after acute myocardial infarction or cardiac surgery. These findings might suggest nutrition-independent regulation of GIP secretion following acute myocardial injury in humans and warrant further investigations.
Nutrient-independent secretion of GLP-1 has been extensively studied in the past. We and others observed that inflammatory stimuli including LPS (lipopolysaccharide), IL-6 (interleukin 6) and IL-1b (interleukin 1b) directly induce GLP-1 secretion from intestinal L-cells through IL-6 signaling [26, 27]. Furthermore, gut intraepithelial lymphocytes were also identified to regulate systemic GLP-1 availability [28]. Consistently mice and patients with acute (sepsis [27] or myocardial infarction [29]) or chronic inflammatory cardiovascular diseases (coronary artery disease (CAD) [30] or heart failure [31]) show strongly increased circulating GLP-1 levels independent of food intake. Elevated GLP-1 levels were independently associated with mortality in patients with sepsis or myocardial infarction [32, 33]. Mechanistic experimental studies suggested upregulated GLP-1 secretion as an endogenous protective counter-regulatory response in terms of cardiovascular and inflammatory diseases [29].
In contrast to GLP-1, nutrition-independent regulation of GIP secretion is not well understood. In mice LPS and IL-1b administration directly induced GIP secretion and patients with peripheral arterial disease (PAD) showed higher circulating GIP levels compared to patients without PAD [21, 34]. However, in contrast to GLP-1, patients with CAD presented with similar circulating GIP levels compared to patients without CAD and GIP was not associated with inflammatory markers [21]. To the best of our knowledge this is the first study investigating endogenous GIP levels in humans following acute myocardial injury or inflammatory challenge. While GLP-1 has been identified and established over the last 10 years as a pleiotropic cardiovascular protective peptide beyond its glucoregulatory role, the relevance of GIP for CVD remains largely unknown. Interestingly, experimental studies found activation of the GIP receptor to exhibit various protective effects in cardiovascular murine disease models (reduction of atherosclerosis, stabilization of atherosclerotic plaques, suppression of cardiac hypertrophy and fibrosis in heart failure models), which does require translational investigations in humans [20,21,22,23, 35]. Recently we found circulating GIP levels in patients with acute myocardial infarction to be associated with favorable cardiovascular prognosis. In these patients higher GIP levels independently predicted reduced cardiovascular mortality [24]. Bioactive GIP and GLP-1 are rapidly inactivated by the enzyme dipeptidyl peptidase 4 (DPP-4). DPP-4 inhibitors improve glucose metabolism and are clinically used for the treatment of diabetes mellitus [3]. However, in several large CV outcome trials DPP-4 inhibitors proved CV safety, but failed to reduce CV endpoints and mortality [36,37,38]. Importantly, DPP-4 inhibitors are not limited to activate GIP and GLP-1 signaling. Next to GIP and GLP-1 DPP-4 has more than 60 other substrates including GLP-2, substance P, neuropeptide Y, stromal cell-derived factor-1α/β (CXCL12), GM-CSF (granulocyte macrophage colony-stimulating factor), CXCL10 and RANTES (Regulated and normal T-Cell-Expressed and Secreted) [39]. Thus, the question how activation of the GIP system directly affects CV prognosis in patients remains open. Due to lack of specificity DPP-4 inhibitors might not be the optimal tool to investigate CV outcome effects of GIP in patients. Future clinical trials with specific GIP receptor agonists are needed to foster our understanding of the gut-heart axis as a yet fairly neglected field of system biology and to elucidate whether the GIP system might open novel therapeutic approaches for the treatment of patients with CVD.
This study has several limitations. The underlying mechanism leading to the reduction of GIP after myocardial infarction or cardiac surgery remains currently unknown. Since GIP levels rose at the late time point after cardiac surgery (second post-operative day) without enteral or parenteral nutrition it appears unlikely that food restriction was the underlying mechanism of reduced GIP levels. Future mechanistic experimental and clinical studies investigating the interplay between GIP and other metabolic hormones are necessary to improve our understanding of GIP regulation in response to acute myocardial injury. We here report lower circulating GIP concentrations in response to myocardial injury. However, this observation does not imply a direct reduction in GIP secretion, since we only measured total GIP (including active and inactive cleaved peptides) and not active GIP (1-42) levels. Lower total GIP levels could also be related to changes in distribution and elimination. Finally, we have no information on food intake in Clinical Study I, which could have affected GIP secretion.
Conclusions
Circulating GIP levels are downregulated in patients with myocardial infarction and following cardiac surgery. These results might suggest nutrition-independent regulation of GIP secretion following myocardial injury in humans. Upcoming studies are needed to elucidate whether GIP might be a novel therapeutic target in CVD.
Availability of data and materials
Data will be provided upon request to the corresponding author.
Abbreviations
- BMI:
-
Body-mass-index
- CAD:
-
coronary artery disease
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- GLP-1:
-
Glucagon-like peptide-1
- GIP:
-
Glucose-dependent insulinotropic peptide
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- ICU:
-
Intensive care unit
- IL-1b:
-
Interleukin 1b
- IL-6:
-
Interleukin 6
- LPS:
-
Lipopolysaccharide
- PAD:
-
Peripheral arterial disease
- RANTES:
-
Regulated and normal T-Cell-Expressed and Secreted
- STEMI:
-
ST-elevation myocardial infarction
- WBC:
-
White blood cells
References
Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4(6):525–36.
Asmar M, Asmar A, Simonsen L, Gasbjerg LS, Sparre-Ulrich AH, Rosenkilde MM, Hartmann B, Dela F, Holst JJ, Bulow J: The gluco- and liporegulatory and vasodilatory effects of glucose-dependent insulinotropic polypeptide (GIP) are abolished by an antagonist of the human GIP receptor. Diabetes 2017, 66(9):2363–2371.
Lehrke M, Marx N: New antidiabetic therapies: innovative strategies for an old problem. Curr Opin Lipidol 2012, 23(6):569–575.
Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24(1):15–30.
Burgmaier M, Liberman A, Mollmann J, Kahles F, Reith S, Lebherz C, Marx N, Lehrke M: Glucagon-like peptide-1 (GLP-1) and its split products GLP-1(9-37) and GLP-1(28-37) stabilize atherosclerotic lesions in apoe(-)/(-) mice. Atherosclerosis 2013, 231(2):427–435.
Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H: Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes 2010, 59(4):1030–1037.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Ryden L et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019.
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018; 392(10157):1519–1529.
Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, Lam CSP, Khurmi NS, Heenan L, Del Prato S, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907.
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019; 381(9):841–851.
Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W: Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab. 1993; 76(4):912–917.
Gasbjerg LS, Helsted MM, Hartmann B, Jensen MH, Gabe MBN, Sparre-Ulrich AH, Veedfald S, Stensen S, Lanng AR, Bergmann NC et al. Separate and combined glucometabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes. 2019; 68(5):906–917.
Vilsboll T, Krarup T, Madsbad S, Holst JJ. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia. 2002; 45(8):1111–1119.
Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W: Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Investig. 1993, 91(1):301–307.
Christensen M, Vedtofte L, Holst JJ, Vilsboll T, Knop FK: Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 2011, 60(12):3103–3109.
Frias JP, Davies MJ, Rosenstock J, Perez Manghi FC, Fernandez Lando L, Bergman BK, Liu B, Cui X, Brown K, Investigators S. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021; 385(6):503–515.
Wilson JM, Lin Y, Luo MJ, Considine G, Cox AL, Bowsman LM, Robins DA, Haupt A, Duffin KL, Ruotolo G: The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: a post hoc analysis. Diabetes Obes Metab. 2022; 24(1):148–153.
Mori Y, Matsui T, Hirano T, Yamagishi SI. GIP as a potential therapeutic target for atherosclerotic cardiovascular disease - a systematic review. Int J Mol Sci. 2020;21(4):1509. https://doi.org/10.3390/ijms21041509
Kahles F, Liberman A, Halim C, Rau M, Mollmann J, Mertens RW, Ruckbeil M, Diepolder I, Walla B, Diebold S et al. The incretin hormone GIP is upregulated in patients with atherosclerosis and stabilizes plaques in ApoE(-/-) mice by blocking monocyte/macrophage activation. Mol Metab. 2018; 14:150–157.
Nagashima M, Watanabe T, Terasaki M, Tomoyasu M, Nohtomi K, Kim-Kaneyama J, Miyazaki A, Hirano T. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia. 2011; 54(10):2649–2659.
Nogi Y, Nagashima M, Terasaki M, Nohtomi K, Watanabe T, Hirano T: Glucose-dependent insulinotropic polypeptide prevents the progression of macrophage-driven atherosclerosis in diabetic apolipoprotein E-null mice. PloS One. 2012; 7(4):e35683.
Kahles F, Ruckbeil MV, Arrivas MC, Mertens RW, Moellmann J, Biener M, Giannitsis E, Katus HA, Marx N, Lehrke M. Association of glucose-dependent insulinotropic polypeptide levels with cardiovascular mortality in patients with acute myocardial infarction. J Am Heart Assoc 2021; 10(13):e019477.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007; 132(6):2131–2157.
Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011; 17(11):1481–1489.
Kahles F, Meyer C, Mollmann J, Diebold S, Findeisen HM, Lebherz C, Trautwein C, Koch A, Tacke F, Marx N et al. GLP-1 secretion is increased by inflammatory stimuli in an IL-6-dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes. 2014; 63(10):3221–3229.
He S, Kahles F, Rattik S, Nairz M, McAlpine CS, Anzai A, Selgrade D, Fenn AM, Chan CT, Mindur JE et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature. 2019; 566(7742):115–119.
Diebold S, Moellmann J, Kahles F, Haj-Yehia E, Liehn EA, Nickel A, Lebherz C, Maack C, Marx N, Lehrke M: Myocardial infarction is sufficient to increase GLP-1 secretion, leading to improved left ventricular contractility and mitochondrial respiratory capacity. Diabetes Obes Metab. 2018; 20(12):2911–2918.
Piotrowski K, Becker M, Zugwurst J, Biller-Friedmann I, Spoettl G, Greif M, Leber AW, Becker A, Laubender RP, Lebherz C et al. Circulating concentrations of GLP-1 are associated with coronary atherosclerosis in humans. Cardiovasc Diabetol. 2013; 12:117.
Hattori A, Kawamura I, Yamada Y, Kanamori H, Aoyama T, Ushikoshi H, Kawasaki M, Nishigaki K, Tamemura G, Minatoguchi S. Elevated plasma GLP-1 levels and enhanced expression of cardiac GLP-1 receptors as markers of left ventricular systolic dysfunction: a cross-sectional study. BMJ Open. 2013; 3(9):e003201.
Lebherz C, Schlieper G, Mollmann J, Kahles F, Schwarz M, Brunsing J, Dimkovic N, Koch A, Trautwein C, Floge J et al. GLP-1 Levels predict mortality in patients with critical illness as well as end-stage renal disease. Am J Med. 2017; 130(7):833-841 e833.
Kahles F, Rückbeil MV, Mertens RW, Foldenauer AC, Arrivas MC, Moellmann J,Lebherz C, Biener M, Giannitsis E, Katus HA, Marx N, Lehrke M. GLP-1 levels predict cardiovascular risk in patients with acute myocardial infarction. Eur Heart J. 2020;41(7):882–889. https://doi.org/10.1093/eurheartj/ehz728.
Kahles F, Meyer C, Diebold S, Foldenauer AC, Stohr R, Mollmann J, Lebherz C, Findeisen HM, Marx N, Lehrke M. Glucose-dependent insulinotropic peptide secretion is induced by inflammatory stimuli in an interleukin-1-dependent manner in mice. Diabetes Obes Metab. 2016; 18(11):1147–1151.
Hiromura M, Mori Y, Kohashi K, Terasaki M, Shinmura K, Negoro T, Kawashima H, Kogure M, Wachi T, Watanabe R et al. Suppressive effects of glucose-dependent insulinotropic polypeptide on cardiac hypertrophy and fibrosis in angiotensin II-infused mouse models. Circ J. 2016; 80(9):1988–1997.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013; 369(14):1317–1326.
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013; 369(14):1327–1335.
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015; 373(3):232–242.
Waumans Y, Baerts L, Kehoe K, Lambeir AM, De Meester I. The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front Immunol. 2015; 6:387.
Acknowledgements
We do thank Kirsten Nassau, Erich Kilger and Klaus G Parhofer (University of Munich, Campus Grosshadern) for sample collection and study support.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by grants from the START-Program of the Faculty of Medicine, RWTH Aachen, the German Society for Cardiology (DGK), the European Foundation for the Study of Diabetes/Novo Nordisk Foundation and the European Research Area Network on Cardiovascular Diseases (ERA-CVD and BMBF, Grant No. JTC-2019, MyPenPath - 01KL2004) to F.K. and grants from the Deutsche Forschungsgemeinschaft (SFB TRR 219 M-03) and Interreg V-A grant EURlipids to ML. NM was supported by grants from the Deutsche Forschungsgemeinschaft (SFB TRR 219 M-03 and M-05) as well as the CORONA Stiftung, Germany. E.P.C.v.d.V was supported by a grant from the Interdisciplinary Center for Clinical Research within the faculty of Medicine at the RWTH Aachen University and NWO-ZonMw Veni (91619053).
Author information
Authors and Affiliations
Contributions
F.K. wrote the first draft of the manuscript, supervised the project, acquired funding, measured patient samples, analyzed data, made figures and tables and provided intellectual input; M. Rau. organized biobanking and acquired patient samples, made tables, organized patient follow-up, performed statistical analyses, edited the manuscript and provided intellectual input; M. Reugels performed statistical analyses, made figures and tables, edited the manuscript and provided intellectual input; A.C.F., R.W.M., M.C.A., J.S., P.I., J.M. and E.P.C.v.V. edited the manuscript and provided intellectual input. N.M. edited the manuscript, provided intellectual input, supervised the project and provided infrastructure. M.L. initiated the project, wrote the first draft of the manuscript, supervised the project, acquired funding, organized biobanking, provided infrastructure, and provided intellectual input as the corresponding author. All authors read and approved final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study protocols and biosampling were approved by the local ethics committee and conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Consent for publication
All authors have read and approved the submission of the manuscript to Cardiovascular Diabetology. Authors permit the Journal to publish these research findings.
Competing interests
The authors do not have any competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kahles, F., Rau, M., Reugels, M. et al. The gut hormone glucose-dependent insulinotropic polypeptide is downregulated in response to myocardial injury. Cardiovasc Diabetol 21, 18 (2022). https://doi.org/10.1186/s12933-022-01454-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01454-3