Abstract
Purpose
A phytochemical- and mineral-rich filtered sugarcane molasses concentrate (FMC), when added to carbohydrate-containing foods as a functional ingredient, lowers postprandial blood glucose and insulin responses. We hypothesised that this beneficial effect would also occur if FMC was administered as an oral supplement taken before a meal.
Methods
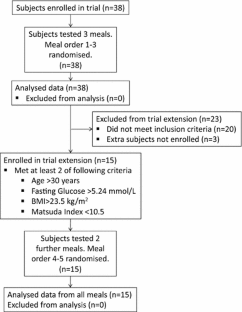
This study measured the postprandial glucose and insulin responses elicited by different doses of FMC administered immediately prior to a standard breakfast to healthy subjects. Each subject was given three or five breakfast meals once, on different days. The composition of the meals was identical, except for the addition of either placebo syrup (test meal 1) or increasing doses of FMC (test meals 2–5).
Results
The plasma glucose concentration curves were similar for the five test meals. Plasma insulin curves were lowered in a dose-dependent manner. Stratifying subjects based on age, BMI and insulin resistance showed greater effects of low doses of FMC on lowering insulin responses in those subjects with potentially greater insulin resistance. When insulin response is standardised to amount of carbohydrate in the meal/dose combination, the reduction in response is linear and inversely proportional to the FMC dose.
Conclusions
FMC shows promise as an agent that can reduce insulin responses and lessen the load on the pancreatic beta cells.



Similar content being viewed by others
References
Liu S, Willett WC (2002) Dietary glycemic load and atherothrombotic risk. Curr Atheroscler Rep 4:454–461
Ludwig DS (2002) The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 287:2414–2423
Cordain L, Eades MR, Eades MD (2003) Hyperinsulinemic diseases of civilization: more than just Syndrome X. Comp Biochem Physiol A: Mol Integr Physiol 136:95–112
Cordain L, Eaton SB, Sebastian A et al (2005) Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 81:341–354
Larsen TM, Dalskov SM, van Baak M et al (2010) Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 363:2102–2113
Goyenechea E, Holst C, Saris WH et al (2011) Effects of different protein content and glycemic index of ad libitum diets on diabetes risk factors in overweight adults: the DIOGenes multicentre, randomised, dietary intervention trial. Diabetes Metab Res Rev 27:705–716
Brand-Miller J, Hayne S, Petocz P, Colagiuri S (2003) Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 26:2261–2267
Thomas D, Elliott EJ (2009) Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. doi:10.1002/14651858.CD006296.pub2
Duarte-Almeida JM, Salatino A, Genovese MI, Lajolo FM (2011) Phenolic composition and antioxidant activity of culms and sugarcane (Saccharum officinarum L.) products. Food Chem 125:660–664
Payet B, Shum Cheong Sing A, Smadja J (2006) Comparison of the concentrations of phenolic constituents in cane sugar manufacturing products with their antioxidant activities. J Agric Food Chem 54:7270–7276
Guimaraes CM, Giao MS, Martinez SS, Pintado AI, Pintado ME, Bento LS, Malcata FX (2007) Antioxidant activity of sugar molasses, including protective effect against DNA oxidative damage. J Food Sci 72:C039–C043
Bravo L (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56:317–333
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81:230S–242S
Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach C, Williamson G (2004) How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr 80:15–21
Dreosti IE (2000) Antioxidant polyphenols in tea, cocoa, and wine. Nutrition 16:692–694
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Kao YH, Chang HH, Lee MJ, Chen CL (2006) Tea, obesity, and diabetes. Mol Nutr Food Res 50:188–210
Scalbert A, Johnson IT, Saltmarsh M (2005) Polyphenols: antioxidants and beyond. Am J Clin Nutr 81:215S–217S
Dembinska-Kiec A, Mykkänen O, Kiec-Wilk B, Mykkänen H (2008) Antioxidant phytochemicals against type 2 diabetes. Br J Nutr 99:ES109–ES117
Avignon A, Hokayem M, Bisbal C, Lambert K (2012) Dietary antioxidants: do they have a role to play in the ongoing fight against abnormal glucose metabolism? Nutrition 28:715–721
Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, Poutenan K (2010) Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci 11:1365–1402
Narita Y, Inouye K (2009) Kinetic analysis and mechanism on the inhibition of chlorogenic acid and its components against porcine pancreas alpha-amylase isozymes I and II. J Agric Food Chem 57:9218–9225
Ishikawa A, Yamashita H, Hiemori M et al (2007) Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J Nutr Sci Vitaminol (Tokyo) 53:166–173
Johnston K, Sharp P, Clifford M, Morgan L (2005) Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett 579:1653–1657
Kim EK, Kwon KB, Song MY et al (2007) Flavonoids protect against cytokine-induced pancreatic beta-cell damage through suppression of nuclear factor kappaB activation. Pancreas 35:e1–e9
Prabhakar PK, Doble M (2009) Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine 16:1119–1126
Jung UJ, Lee MK, Park YB, Kang MA, Choi MS (2006) Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol 38:1134–1145
Jung UJ, Lee MK, Jeong KS, Choi MS (2004) The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J Nutr 134:2499–2503
Lin CL, Huang HC, Lin JK (2007) Theaflavins attenuate hepatic lipid accumulation through activating AMPK in human HepG2 cells. J Lipid Res 48:2334–2343
Wright AG, Ellis TP, Ilag LL (2014) Filtered molasses concentrate from sugar cane: natural functional ingredient effective in lowering the glycaemic index and insulin response of high carbohydrate foods. Plant Foods Hum Nutr 69:310–316
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
Lee BM, Wolever TMS (1998) Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: comparison with white bread. Eur J Clin Nutr 52:924–928
Zhang W, Kim D, Philip E et al (2013) A multinational, observational study to investigate the efficacy, safety and tolerability of acarbose as add-on or monotherapy in a range of patients: the Gluco VIP study. Clin Drug Investig 33:263–274
Ma J, Stevens JE, Cukier K et al (2009) Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 32:1600–1602
Wu T, Bound MJ, Zhao BR et al (2013) Effects of a d-xylose preload with or without sitagliptin on gastric emptying, glucagon-like peptide-1, and postprandial glycemia in type 2 diabetes. Diabetes Care 36:1913–1918
Welsch CA, Lachance PA, Wasserman BP (1989) Dietary phenolic compounds: inhibition of Na+-dependent d-glucose uptake in rat intestinal brush border membrane vesicles. J Nutr 119:1698–1704
Jung KH, Choi HS, Kim DH et al (2008) Epigallocatechin gallate stimulates glucose uptake through the phosphatidylinositol 3-kinase-mediated pathway in L6 rat skeletal muscle cells. J Med Food 11:429–434
Eid HM, Martineau LC, Saleem A et al (2010) Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol Nutr Food Res 54:991–1003
Park CE, Kim MJ, Lee JH et al (2007) Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp Mol Med 39:222–229
Cao H, Polansky MM, Anderson RA (2007) Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch Biochem Biophys 459:214–222
Kwon DY, Jang JS, Lee JE, Kim YS, Shin DH, Park S (2006) The isoflavonoid aglycone-rich fractions of Chungkookjang, fermented unsalted soybeans, enhance insulin signaling and peroxisome proliferator-activated receptor-gamma activity in vitro. BioFactors 26:245–258
Montagut G, Onnockx S, Vaque M et al (2010) Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin. J Nutr Biochem 21:476–481
Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK (2002) Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem 277:34933–34940
Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA (2002) Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 283:E1135–E1143
Mertens-Talcott SU, Percival SS (2005) Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Lett 218:141–151
Kim D-O, Jeong SW, Lee CY (2003) Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem 81:321–326
Lamaison JL, Carnet A (1991) Teneurs en principaux flavonoides des fleurs de Crataegeus monogyna Jacq et de Cratageus Laevigata (Poiret D.C) en Fonction de la vegetation. Plantes Med Phytother 25:12–16
Cao G, Alessio H, Cutler R (1993) Oxygen-radical absorbance capacity (ORAC) assay for antioxidants. Free Radical Biol Med 14:303–311
Acknowledgments
The authors would like to thank Fiona Atkinson from Sydney University Glycaemic Index Research Service for valuable insight and assistance with this study. The authors would also like to thank Michael Cowley, Ralph DeFronzo, Seema Gulati, Edward Horton, Chris Newguard and Gerald Shulman for their discussions around this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
This study was conducted under contract by the Sydney University Glycaemic Index Research Service and financed by Horizon Science. Horizon Science retained the services of the Baker IDI Heart and Diabetes Institute for the scientific input of Peter Clifton towards this study and manuscript.
Rights and permissions
About this article
Cite this article
Ellis, T.P., Wright, A.G., Clifton, P.M. et al. Postprandial insulin and glucose levels are reduced in healthy subjects when a standardised breakfast meal is supplemented with a filtered sugarcane molasses concentrate. Eur J Nutr 55, 2365–2376 (2016). https://doi.org/10.1007/s00394-015-1043-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1043-6




